One way of changing the crystal structure is to do so without disrupting the relative order of the atoms. This can be done by generating the unit cell of ferrite by a homogeneous deformation of the parent γ. In thisdisplacive mechanism, the overall shape of the sample must change in a manner consistent with the change in crystal structure. When this shape deformation occurs in the bulk of a polycrystalline steel, its accommodation leads to a lot of strain energy. This energy can be minimised if the ferrite adopts a thin-plate shape. Since transformation occurs by a deformation, the atoms maintain the sequence which existed in the parent phase. There is therefore, no change in the chemical composition during transformation. There is also a one-to-one atomic correspondence between the ferrite and austenite, which is the basis of the shape memory effect.
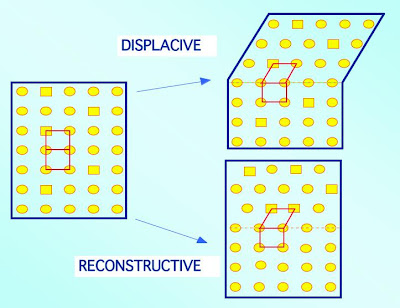
The change in crystal structure can also be achieved in effect by breaking the bonds in the austenite and rearranging the atoms into the structure of ferrite whilst maintaining the overall shape. This requires atoms to diffuse over distances comparable to the size of the transformation product. Thus, although the strain energy associated with displacive transformations is avoided, this reconstructive mechanism can only occur at temperatures where atoms are sufficiently mobile.

Given that atoms are mobile, certain species which are more soluble in a particular phase (α or γ) will tend to migrate preferentially into that phase, leading to a difference in the chemical composition between α and γ. The atomic correspondence between the parent and product phases is lost in a reconstructive transformation. The shape of the transformation product is either determined by growth circumstances, or as equilibrium is approached, by a minimisation of the overall interfacial energy per unit volume.
No comments:
Post a Comment